

We recommend using aĪuthors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Use the information below to generate a citation. Then you must include on every digital page view the following attribution: If you are redistributing all or part of this book in a digital format, Then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a print format, Want to cite, share, or modify this book? This book uses the Example 21.4 shows how we can identify a nuclide by balancing the nuclear reaction. For instance, we could determine that 8 17 O 8 17 O is a product of the nuclear reaction of 7 14 N 7 14 N and 2 4 He 2 4 He if we knew that a proton, 1 1 H, 1 1 H, was one of the two products. If the atomic number and the mass number of all but one of the particles in a nuclear reaction are known, we can identify the particle by balancing the reaction. The sum of the charges of the reactants equals the sum of the charges of the products.The sum of the mass numbers of the reactants equals the sum of the mass numbers of the products.Nuclear reactions also follow conservation laws, and they are balanced in two ways: A balanced nuclear reaction equation indicates that there is a rearrangement during a nuclear reaction, but of nucleons (subatomic particles within the atoms’ nuclei) rather than atoms. Balancing Nuclear ReactionsĪ balanced chemical reaction equation reflects the fact that during a chemical reaction, bonds break and form, and atoms are rearranged, but the total numbers of atoms of each element are conserved and do not change.
Due to the much larger energy differences between nuclear energy shells, gamma rays emanating from a nucleus have energies that are typically millions of times larger than electromagnetic radiation emanating from electronic transitions. Gamma rays are a type of high energy electromagnetic radiation produced when a nucleus undergoes a transition from a higher to a lower energy state, similar to how a photon is produced by an electronic transition from a higher to a lower energy level. −1 0 e + +1 0 e ⟶ γ + γ −1 0 e + +1 0 e ⟶ γ + γĪs seen in the chapter discussing light and electromagnetic radiation, gamma rays compose short wavelength, high-energy electromagnetic radiation and are (much) more energetic than better-known X-rays that can behave as particles in the wave-particle duality sense.

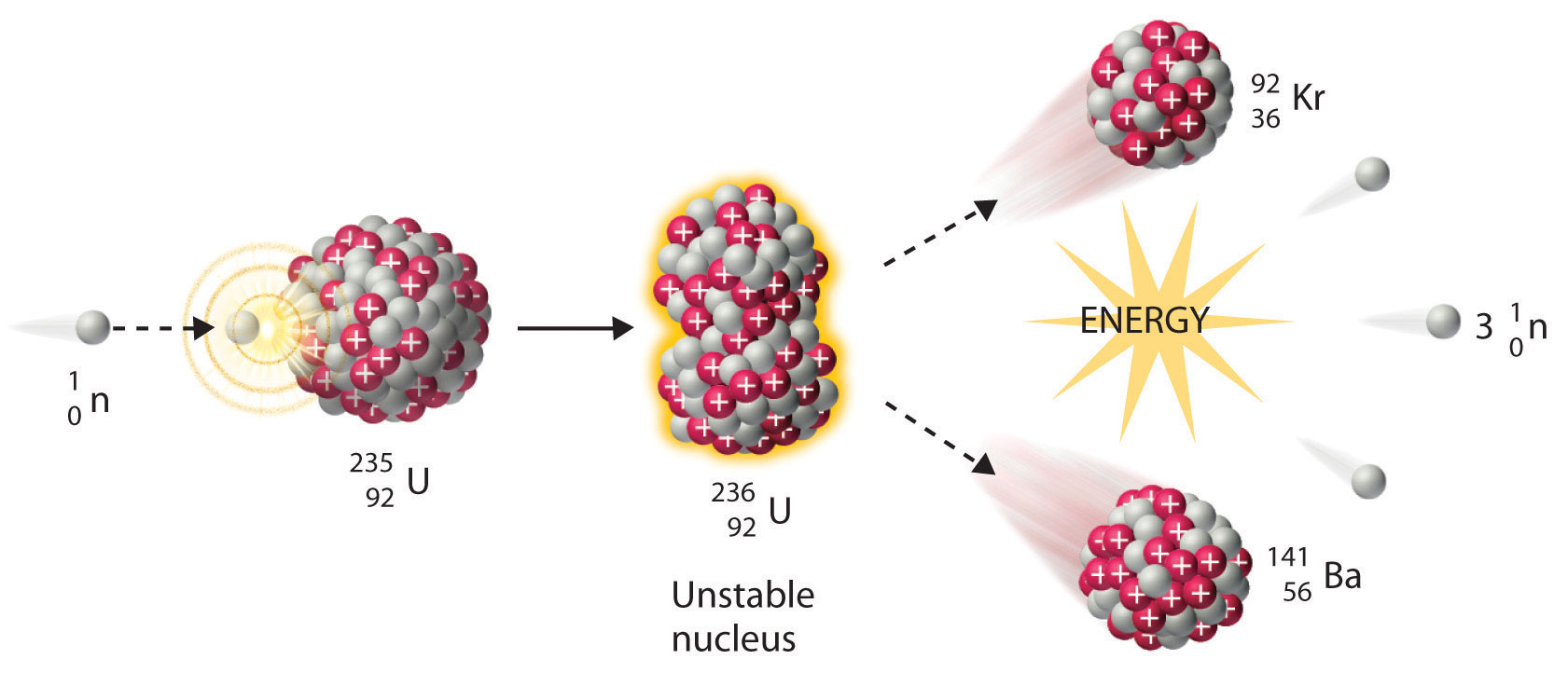
This works because, in general, the ion charge is not important in the balancing of nuclear equations. For example, an alpha particle is a helium nucleus (He) with a charge of +2 and a mass number of 4, so it is symbolized 2 4 He. The subscripts and superscripts are necessary for balancing nuclear equations, but are usually optional in other circumstances. Positrons ( +1 0 e, ( +1 0 e, also represented by the symbol +1 0 β ) +1 0 β ) are positively charged electrons (“anti-electrons”). Beta particles ( −1 0 β, ( −1 0 β, also represented by the symbol −1 0 e ) −1 0 e ) are high-energy electrons, and gamma rays are photons of very high-energy electromagnetic radiation. Alpha particles ( 2 4 He, ( 2 4 He, also represented by the symbol 2 4 α ) 2 4 α ) are high-energy helium nuclei. Protons ( 1 1 p, ( 1 1 p, also represented by the symbol 1 1 H ) 1 1 H ) and neutrons ( 0 1 n ) ( 0 1 n ) are the constituents of atomic nuclei, and have been described previously. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in Figure 21.4. Many entities can be involved in nuclear reactions. To describe a nuclear reaction, we use an equation that identifies the nuclides involved in the reaction, their mass numbers and atomic numbers, and the other particles involved in the reaction. Identify common particles and energies involved in nuclear reactionsĬhanges of nuclei that result in changes in their atomic numbers, mass numbers, or energy states are nuclear reactions.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
